Preparation of Ethers
Preparation of Ethers: Overview
In this topic, we will learn how to prepare ethers in the laboratory. It discusses their formation by dehydration of alcohols, Williamson synthesis and other methods. It also includes the mechanism of action of reagents.
Important Questions on Preparation of Ethers
-haloethers on reacting with a _____ will form the higher ethers.
Identify the reagent for the given reaction:
Write the reaction for the preparation of methoxyethane from Grignard reagent.
Write an example for the preparation of higher ethers from -haloethers.
Identify the ether () formed from the below reaction:
Identify the reagent used for the below reaction:
Write the reaction for the preparation of diethyl ether using dry silver oxide.
Methoxy ethane can be prepared by Williamson's ether synthesis. When Sodium _____ reacts with Methyl iodide in the presence of dry ether, we get our desired product.
The major product of the following reactions is
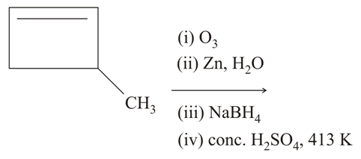
How will you effect following two step conversions ethylene into ethyl methyl ether?
How is diethyl ether prepared by continuous etherification process? Why is the process so called?
Write the chemical equation for Williamson synthesis of -Ethoxy--methylpentane starting from Ethanol and -Methylpentan--ol.
What are ethers? How are ethers classified?
Write chemical equation for the preparation of following ether as major product by Williamson synthesis.
-Methoxy--nitrobenzene.
Write chemical equation for the preparation of following ether as major product by Williamson synthesis.
Ethyl phenyl ether.
Write chemical equations for the preparation of following ether as major product by Williamson synthesis.
Benzyl isopropyl ether
Write the general formula of ether.
The following reaction is:
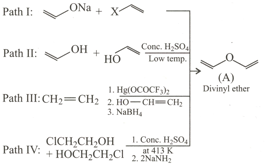
Which of the following paths is/are feasible for the preparation of ether (A) ?
Which of the following alcohols gives the best yield of dialkyl ether on being heated with a trace of sulphuric acid?
